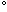 F, while a carp, in contrast, can survive in as little as 3 parts per million at temperatures in excess of 85
F, while a carp, in contrast, can survive in as little as 3 parts per million at temperatures in excess of 85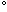 F.
F.
Obviously, it is dissolved oxygen that is absolutely necessary for aerobic life in water. Dissolved carbon dioxide in water, on the other hand, is needed for the growth of quality microscopic algae, which produce oxygen and food at the base of the food chain. Warmer water drives off these vital gases.
The biological ramifications of cold and warm water are countless. One situation familiar to most involves the high profile fishes such as the trouts, which require cold water and high oxygen concentrations for their metabolism. Lake trout, for example, require in excess of 11 parts per million dissolved oxygen and like to feed at about 48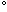 F, while a carp, in contrast, can survive in as little as 3 parts per million at temperatures in excess of 85
F, while a carp, in contrast, can survive in as little as 3 parts per million at temperatures in excess of 85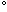 F.
F.
Higher temperatures decrease the solubility of essential gases while increasing the solubility of plant nutrients like nitrates and phosphates. These materials are the basic ingredients of fertilizer and cause prolific plant growth in water, the likes of which are undesirable by both man and the existing ecosystems in Cayuga and Seneca Lakes.
In 1998, all across the Finger Lakes, there was a noticeable increase in the growth of rooted aquatic vegetation as well as an increase in the magnitude and duration of microscopic algae blooms. Higher average air and water temperatures, greater sun intensity and the absence of the usual ice covers at the ends of the lakes allowed them to warm up sooner this year. (Ice or slush formation and melting accommodates 80 times as much heat as liquid water). As a result, the average temperature of the lakes was much warmer in early to middle 1998 causing precocious and prolific activity of all types of aquatic life. This natural event should illustrate to all that coldness is a price you pay for good quality water for both humans and fish.
An old limnological rule of thumb is that for every 10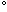 C (18
C (18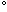 F) increase in water temperature there is a doubling of the metabolic activity in the water. This means that with a temperature increase, there is more growth, more eating, more excreting, more reproduction, more algae blooms, more decomposition, etc. Unfortunately, there is a threshold above which quality aerobic life can no longer exist in warm waters. Since oxygen is needed for complete breakdown of organic matter, the decrease in dissolved oxygen that is associated with the higher water temperatures often cannot accommodate the decomposition that is necessary to keep up with the recycling of such growth.
F) increase in water temperature there is a doubling of the metabolic activity in the water. This means that with a temperature increase, there is more growth, more eating, more excreting, more reproduction, more algae blooms, more decomposition, etc. Unfortunately, there is a threshold above which quality aerobic life can no longer exist in warm waters. Since oxygen is needed for complete breakdown of organic matter, the decrease in dissolved oxygen that is associated with the higher water temperatures often cannot accommodate the decomposition that is necessary to keep up with the recycling of such growth.
In short, the complications that can arise in lake ecology by higher water temperatures are very intricate. Most aquatic organisms have temperature range specifications and preferences. Obviously though, highly desirable fish, quality microscopic algae and even humans do not benefit by higher average water temperatures. Indeed, warmer water temperatures will encourage the eutrophication (aging) of a lake.
The heat of the earth, sunlight, and unnatural industry are the most common sources of heat in a body of water. Some sources can be controlled and others cannot.
CLDF 1998