Will Our Lakes Freeze Over Completely?
by Mel Russo
When the air temperature gets unusually cold for several weeks, there is always talk of Seneca and or Cayuga Lake freezing over completely. Yet, it is very rare for this to happen. The rarity of a total lake freeze-over is due to the interaction of four physical factors associated with the lakes: their great depths, their extensive surface area, the frequent Finger Lakes winds and industrial heat discharge.
In the fall, when surface water temperatures start to decrease, the surface waters get more dense and they sink. This factor coupled with vigorous wind action, causes a mixing of the waters until there is nearly a uniform temperature from the surface to the bottom of the lake. Eventually this temperature will become 39.2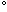 F. When water gets colder than this, it becomes less dense and remains nearer the surface. The water may eventually reach its least density at 32
F. When water gets colder than this, it becomes less dense and remains nearer the surface. The water may eventually reach its least density at 32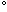 F. At this temperature water becomes slush and this material floats, thereby allowing lakes to freeze from the top down.
F. At this temperature water becomes slush and this material floats, thereby allowing lakes to freeze from the top down.
In wintertime on Seneca and Cayuga Lakes, the wind normally mixes the cooler surface waters with the warmer deeper waters resulting in heat transfer to the upper layers of water. Therefore, the lakes rarely freeze over completely, except where they are consistently shallow. In addition, power plants and other industries are constantly removing cool, 39.2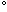 F water and discharging much warmer water at the surface. This discharge adds significant heat to the lakes and inevitably maintains a certain area of open water. However, if we get a short period of calm, together with extremely cold temperatures, we may be able to tell of a year when the lake froze over all the way!
F water and discharging much warmer water at the surface. This discharge adds significant heat to the lakes and inevitably maintains a certain area of open water. However, if we get a short period of calm, together with extremely cold temperatures, we may be able to tell of a year when the lake froze over all the way!
Beware! Stay off the ice in areas that don't normally freeze or you may become a legend yourself!
Prepared by the
Cayuga Lake Defense Fund (CLDF).
For more information, Call: 275-9054 or 272-7914
or email
info@cldf.org
CLDF 1998
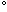 F. When water gets colder than this, it becomes less dense and remains nearer the surface. The water may eventually reach its least density at 32
F. When water gets colder than this, it becomes less dense and remains nearer the surface. The water may eventually reach its least density at 32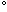 F. At this temperature water becomes slush and this material floats, thereby allowing lakes to freeze from the top down.
F. At this temperature water becomes slush and this material floats, thereby allowing lakes to freeze from the top down.
